|
 |
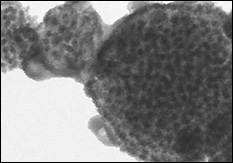
The carbon-tin nanocomposite particles take the form of multiple 10nm wide tin spheres embedded in 200-500nm wide carbon spheres. |
|
A technique developed at the Clark School for creating a durable tin-carbon nanocomposite battery anode has been published in Nano Letters and highlighted in Chemical and Engineering News (C&EN), the weekly news magazine of the American Chemical Society (ACS). The new anode material, which is capable of storing two and a half times the energy graphite anodes can, is designed for use in fast-charging, high energy capacity batteries.
The development is the result of collaboration among the research groups of University of Maryland Energy Research Center members Chunsheng Wang (Department of Chemical and Biomolecular Engineering) and Michael Zachariah (Departments of Mechanical Engineering and Chemistry & Biochemistry).
In her C&EN feature, Katherine Bourzac describes the new manufacturing technique that makes it all possible: While tin anodes can hold and provide much more energy than graphite, they eventually break down from the strain they experience during repeated cycles of charging and discharging. An anode made of smaller, nanoscale tin particles reduces the strain, and the addition of carbon to the tin provides additional strength and support. Creating consistently sized particles of the carbon-tin composite, however, had been difficult due to the low melting point of tin. Wang, Zachariah and their teams found a way around the problem by exposing an aerosolized solution containing the tin and carbon sources to 900° C (1652° F) heat. The the combination of tiny, consistent droplets and extremely high heat cause the necessary reactions take place in less than a second and leave behind a non-clumping nanocomposite powder that quickly cools.
In subsequent experiments, a battery constructed with a tin-carbon anode remained stable, maintained its storage capacity, and exhibited a faster discharge/recharge cycle than currently available cell phone batteries.
For More Information:
"Tin-Carbon Nanocomposite Enables Fast-Charging Battery." Katherine Bourzac. C&EN, published online 10 January 2013.
"Uniform Nano-Sn/C Composite Anodes for Lithium Ion Batteries." Yunhua Xu, Qing Liu, Yujie Zhu, Yihang Liu, Alex Langrock, Michael R. Zachariah, and Chunsheng Wang. Nano Lett., published online 2 January 2013. DOI: 10.1021/nl303823k. Abstract »
Visit Professor Wang's web site »
Visit Professor Zachariah's web site »
Visit Dr. Zhang's web site »
Related Articles:
Building Energy Innovation in Maryland
Wachsman and group overcome high resistance, low capacity solid-state battery barriers
New Course | ENME 808: Batteries - Operation, Modeling and Reliability
A Battery Made of Wood?
January 10, 2013
|

